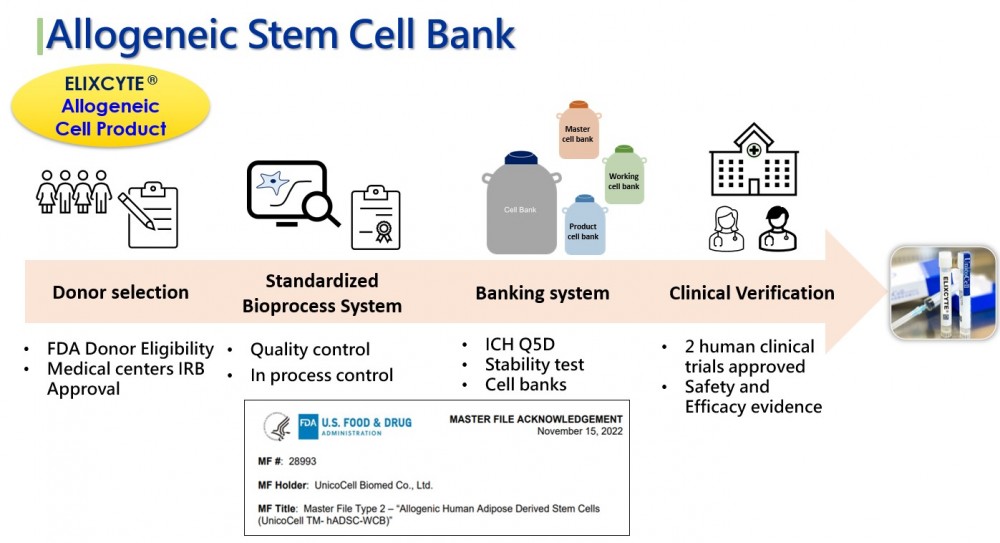
With over 10 years of experiences on research and development of stem cell technology, UnicoCell has designed a robust build-in quality system in our facility which has been certified by governments and accreditation agent:
*The only cell bank in Taiwan with FDA Master File (MF) registration and approval
* From donor qualification to cell bank establishment, all processes strictly adhere to ICH guidelines
* Passed comprehensive safety and viral testing
* Good Tissue Practice laboratory and Human Organ Storage facility by Taiwan FDA
* Overseas Cell Processing Center by PMDA of Japan
* ISO 17025 laboratory by Taiwan Accreditation Foundation, member of International Laboratory Accreditation Cooperation
To accomplish our vision of promoting widespread application of cell technology, we keep pushing forward to enhance our quality system and proprietary process in the new PIC/S GMP factory to scale up production capacity and to ensure the consistency of quality.
The new stem cell drug " ELIXCYTE®" developed by UnicoCell has successively obtained the approval of Investigational New Drug (IND) Application by Taiwan FDA (CT21, CT31) and US-FDA (CT31) to be tested in clinical trials.
Derived from UnicoCell's allogeneic cell bank, ELIXCYTE® is currently undergoing Phase 3 clinical trials for knee osteoarthritis, while Phase 1/2 clinical trials for chronic kidney disease have been completed and are now in the data analysis stage. ELIXCYTE® not only rigorously oversees its source materials but also maintains a stringent quality management system. Its safety and efficacy have been recognized by the U.S. FDA's Master File, positioning it for a streamlined drug approval process in the future. These outstanding characteristics position UnicoCell's allogeneic cell bank as a leading source for clinical trials and academic research.


Up to date, UnicoCell is conducting multiple clinical and preclinical development programs for ELIXCYTE®, an allogeneic stem cell–based investigational new drug.
Among these programs, the indication for degenerative knee osteoarthritis has completed patient enrollment in a Phase III clinical trial and is currently undergoing ongoing follow-up.
For the chronic kidney disease (CKD) indication, Phase I/II clinical trials have been completed, and the program has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA).
In addition to the above clinical trials, ELIXCYTE® continues to expand into additional potential indications. The company has initiated preclinical studies in other aging-related degenerative diseases to evaluate safety and therapeutic potential, which will serve as the basis for future clinical development.

 ELIXCYTE® treatment for knee osteoarthritis
ELIXCYTE® treatment for knee osteoarthritis

Mechanism of actions of ELIXCYTE®
1. Immune regulation, suppression of inflammation
2. Promote the proliferation of chondrocytes
3. Protect and repair damaged cartilage cells to maintain normal functions
The pathological feature of osteoarthritis is continuous deterioration and irreversible cartilage degradation, which causes pain, stiffness, and decreased function, and is one of the main causes of inconvenience and obstacles. The current treatment strategies for osteoarthritis are mainly to relieve pain and control symptoms. According to the cell model pharmacological research and animal knee osteoarthritis model efficacy studies, ELIXCYTE® has been proved that ELIXCYTE® has significant functions including immune regulation, suppression of inflammation, promotion of the proliferation of chondrocytes and maintenance of the normal function of damaged chondrocytes, and can relief the pain response and maintenance normal cartilage structure.
 ELIXCYTE® treatment for chronic kidney disease
ELIXCYTE® treatment for chronic kidney disease

Mechanism of actions of ELIXCYTE®
1. Immune regulation, suppression of inflammation
2. Inhibition of fibrosis
3. Anti-oxidation and protection of kidney cells
4. Promotion of angiogenesis
The kidneys undergo irreversible damages due to long-term inflammation, diabetes, high blood pressure, or structural damage to the kidney itself, leading to the gradual disappearance of normal kidney functions. It is called chronic kidney disease (CKD). Current treatment for chronic kidney disease mainly focuses on the control of the primary disease and related complications to slow down the deterioration of kidney function. Our pharmacological studies revealed that ELIXCYTE® has significant immune regulation, inhibition of inflammation, protection of kidney cells damaged by oxidative stress, and promotion of angiogenesis. A significant improvement in inflammation and improvement in fibrosis can be observed in kidney tissues in our chronic kidney disease animal model studies.

Clinical and Translational Science, 26 Nov 2025
The results showed that patients in the standard-of-care treatment group exhibited a mean annual decline in kidney function, as measured by estimated glomerular filtration rate (eGFR), of 18.37%, as illustrated in the corresponding figure. In contrast, patients treated with ELIXCYTE®, a stem cell therapy, showed a substantially smaller mean annual decline in eGFR of 3.97%. The difference between the two groups reached statistical significance (p<0.05). Notably, the dosage group demonstrating the best kidney function stability in the clinical trial showed a 3.14% increase in eGFR after one year of follow-up.
Cytotherapy, 1 May 2022
Study the Mechanism of Action of Elixcyte®, an Allogeneic Stem Cell Product, on Osteoaritis
Based on the pharmacological mechanisms of ELIXCYTE® obtained from our pre-clinical studies, for osteoarthritis treatment, our stem cell products show good immune regulation, anti-inflammatory properties, as well as the characteristics of promoting chondrocyte proliferation and protecting chondrocytes.
Journal of Cellular and Molecular Medicine, 12 Apr 2022
This phase 1 trial demonstrated single-dose intravenous ELIXCYTE® was well tolerated in moderate-to-severe CKD patients, with an increase in eGFR was observed in 7 out of 12 subjects (58%) at Week 24 and in 6 of 12 subjects (50%) by Week 48, and its preliminary efficacy warrants future studies.
Stem Cell Research & Therapy, 30 Oct 2021
The changes of the primary endpoint, WOMAC pain score at Week 24, showed significant differences in all ELIXCYTE® groups. The significant differences of visual analog scale (VAS) pain score and Knee Society Clinical Rating System (KSCRS) functional activities score at Week 48 after ELIXCYTE® administration suggested the potential of ELIXCYTE® in the longer duration of the effectiveness compared to HA group. In conclusion, ELIXCYTE® for knee osteoarthritis treatment was effective, safe, and well-tolerated. The efficacy results were showed that ELIXCYTE® conferred the earlier onset of reductions in pain scores and improvements in functional scores than HA group.